TNSET 2018 Notification, Exam Date and time schedule, Online Application Form, Syllabus, Hall tickets, results and other details are published at tnsetexam2018mtwu.in. As a Nodal Agency of the UGC, the Mother Teresa Women’s University, Kodaikanal announces the conduct of the State Eligibility Test (TNSET 2018) on 04.03.2018 (Sunday) for determining the eligibility for Assistant Professor in Tamil Nadu Universities and Colleges. The University will conduct TNSET 2018 in 26 Subjects at 11 Examination Centers across the State.
Candidates who qualify for TNSET 2018 will be governed by the eligibility criteria stipulated for the Assistant Professors of concerned Universities / Colleges / Institutions (Government/ Aided /Private) of Tamil Nadu State.
Income and Community Certificates:
Income Certificate and Community Certificate produced as supporting evidence will be accepted, provided the same is duly issued by the concerned competent authorities. The candidates belonging to OBC with less than 8 lakhs of income per annum will be classified under Non Creamy Layer. Those with annual income of 8 Lakhs and above will be classified under Creamy Layer as per the norms of the Government of Tamil Nadu.
TNSET 2018 Notification, Exam Date and Time Schedule, Online Application Form
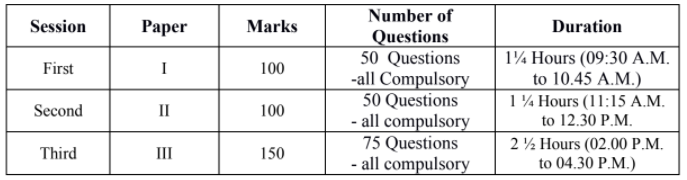
SCHEME AND DATE OF TEST:
The Test will consist of three papers. All the three papers will consist of only objective type questions/ MCQs and will be held on 04.03.2018 (Sunday) in three separate sessions as follows:
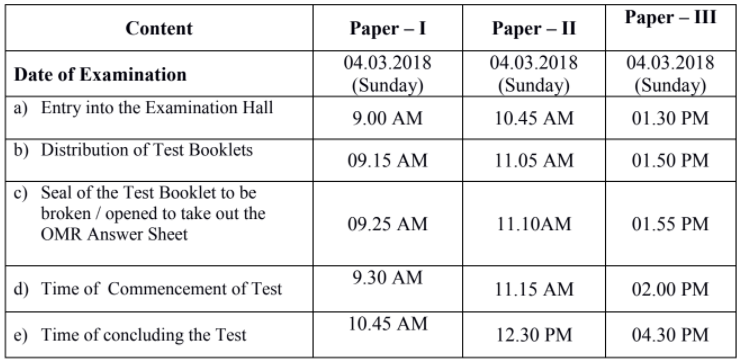
TIME SCHEDULE
TNSET 2018 ELIGIBILITY:
1. Candidates who have secured at least 55% of marks (without rounding off) for General/ OBC (belonging to Creamy Layer) Category and 50% for OBC(belonging to Non – Creamy Layer)/ Scheduled Caste (SC), Scheduled Tribe (ST), Persons with Disability (PwD-VI/HI/PH), in Master’s Degree or equivalent examination from Universities/Institutions recognized by UGC New Delhi, in subjects like Sciences, Humanities (including languages), Social Sciences, Computer Science & Applications, Electronic Sciences, Library & Information Science, are eligible to appear for this examination.
2. Candidates who have appeared or will be appearing at the Master Degree(final year) examination and whose result is still awaited or candidates whose qualifying examinations have been delayed may also appear for this examination. However, such candidates will be admitted provisionally and for those who clear the TNSET 2018 examination will have to qualify within two years from the date of TNSET 2018 Examination, failing which they will be treated as disqualified.
3. Candidates shall appear for TNSET 2018 examination in their relevant subject of their Post-Graduation only. In case the Candidate did not find the subject of their Post Graduation in the list of TNSET 2018 subjects, the Candidate may appear in UGC-NET / UGC-CSIR NET which is held twice a year.
4. Candidates other than General Category are required to mention their Social Status in the Online Application Form. Candidates may note that they should satisfy the eligibility conditions with reference to the documents such as Community Certificate and Certificates related to PwD (VI/HI/PH), OBC(Creamy /Non Creamy Layer), when they upload their applications. The Income Certificate and Community Certificate uploaded by the candidates must be issued by the competent authorities.In case any of the Certificates mentioned above is found to be invalid by the TNSET 2018 authority at any stage, their candidature will be cancelled and they shall be liable for legal action.
5. Candidates should note that their candidature is provisional. The mere fact that an admission card has been issued to a candidate will not imply that the TNSET 2018 authority has accepted his/her candidature. Candidates may note that candidature will be deemed final, only upon verification of eligibility conditions.
Age Limit:
There is no upper age limit for applying for TNSET2018 examination.
How to Apply For TNSET 2018
1. The candidates must read the conditions of eligibility carefully and must satisfy themselves regarding their eligibility for the TNSET 2018 before filling the Online Application Form.
2. Submission of Online Application is mandatory. Hard copies of application form will not be accepted by Mother Teresa Women’s University.
3. Application Form must be complete in all respects as per the Notification.
4. All incomplete Application Forms will be rejected.
5. Applications submitted on any other format will not be accepted.
6. Application submitted without the prescribed fee will be rejected.
7. Before applying Online, the candidates must possess the scanned images as below:-
- Passport size photograph in JPG format of the file size prescribed in the online application websitewww.tnsetexam2018mtwu.in.
- Signature of the candidate in JPG format of the file size prescribed in the online application website www.tnsetexam2018mtwu.in.
- Income Certificate, Community Certificate and PwD Certificate issued by Competent authorities are to be uploaded in PDF format of the file size prescribed in the online application website www.tnsetexam2018mtwu.in.
Examination Fee:
- General/ OBC Creamy Layer: Rs. 1500/-
- OBC (Non Creamy Layer)/ as per the list available on website: www.tnpsc.gov.in : Rs. 1250/
- SC/ST/PwD- VI,HI,PH : Rs. 500/-
TNSET 2018 HALL TICKET
It may be noted that the Hall Ticket will be uploaded on the website: www.tnsetexam2018mtwu.in. No Hall Ticket will be sent to the candidates by post. The candidates should download their Hall Tickets from the above website to ascertain their venue of the Exam. No Candidate will be allowed to appear at the Examination Centre other than that allotted to him/her in the Hall Ticket.
SYLLABUS :
Syllabus for TNSET 2018 examination will be the same as in UGC/CSIR-NET. Mother Teresa Women’s University, Kodaikanal will not send the syllabus to the candidates individually. Syllabi for all TNSET subjects can be downloaded from UGC website: www.ugc.ac.in.
TNSET 2018 Results
The result of the TNSET 2018 Examination held on 04.03.2018 will be published on the Website: www.tnsetexam2018mtwu.in for authenticated view by candidates who have been admitted for the exam. Only qualified candidates will receive intimation through SMS.

320-x100(1).gif)